News Excerpt:
Analyses of ancient rocks and minerals like Zircon to determine the composition of Earth’s early environment revealed that the planet most likely had the conditions for life only about 600 million years after its birth.
Key takeaways from the study:
- The study, published in the journal Nature Geoscience, found evidence of fresh water and dry land interactions as far back as 4 billion years ago, suggesting the presence of conditions conducive to the emergence of life.
- The widespread interaction of fresh water and land on the early Earth may have created conditions conducive to the emergence of life, referred to as the "water cycle."
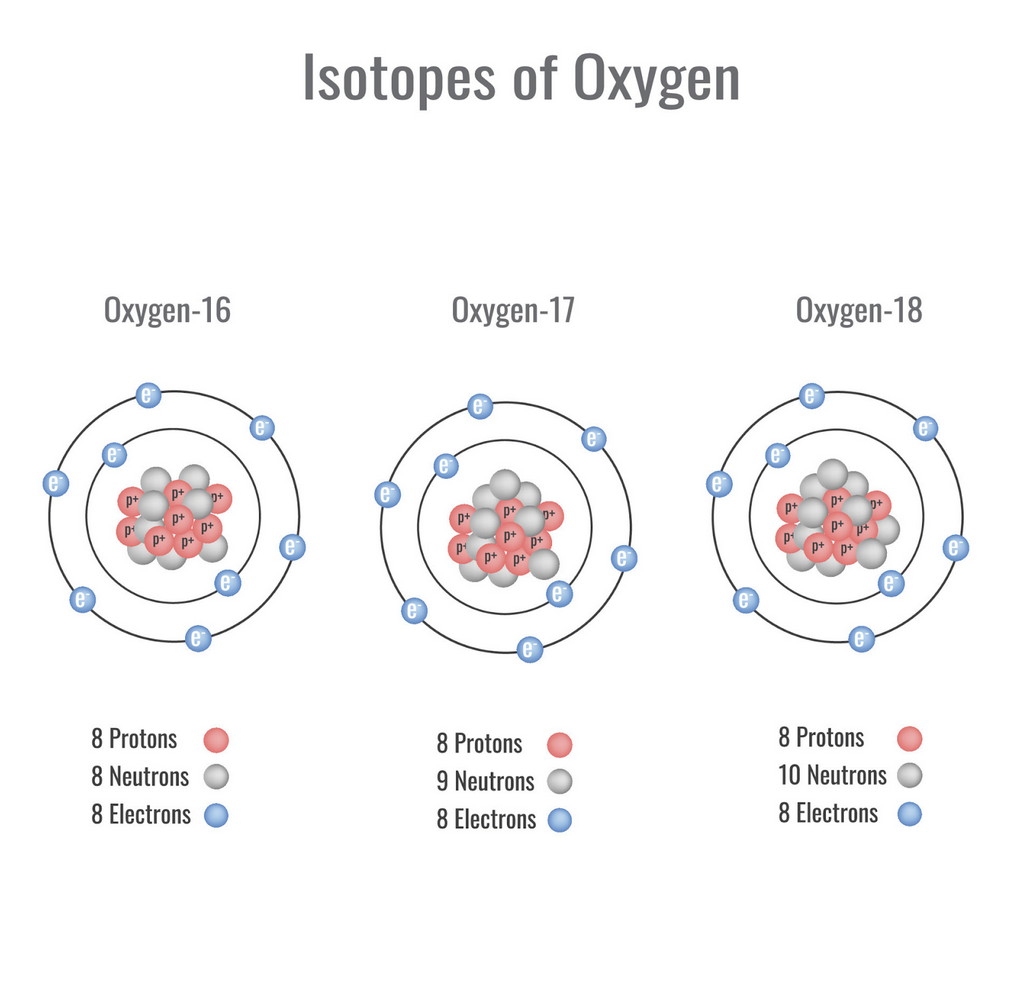
- The researchers studied oxygen isotopes in zircon crystals from the Jack Hills in Western Australia, which can be up to 4.4 billion years old, providing insights into the early Earth.
- They found unusually light isotopic signatures as far back as four billion years ago, such light oxygen isotopes indicate that water and rocks interacted with each other several kilometers below the Earth’s surface.
- Fresh water and emerged land are vital components of the water cycle, as solely salty ocean water wouldn't sustain life emergence.
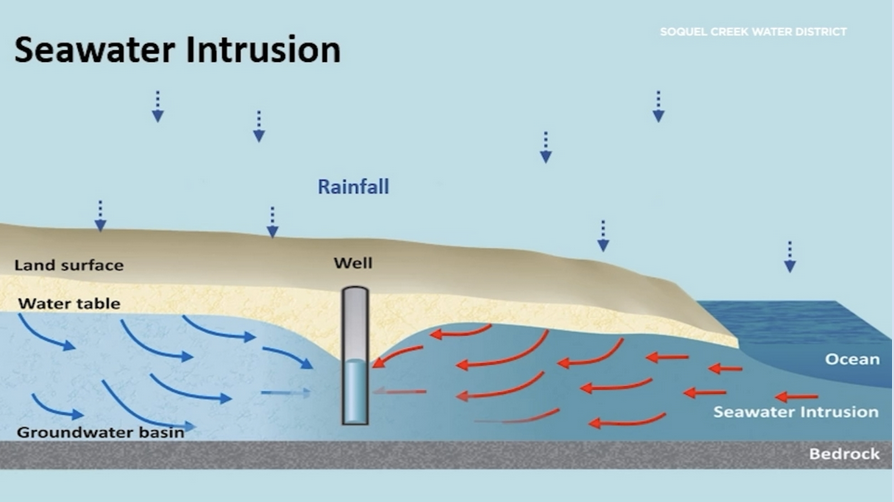
- If all land is submerged, only salty ocean water remains, as salty water naturally seeks to infiltrate land, known as seawater intrusion.
- This evidence challenges the existing theory that Earth was completely covered by an ocean 4 billion years ago. However, according to the paper, finding direct evidence of life during that period remains a challenge.
|
About Seawater intrusion: As sea levels rise along the coasts, saltwater can move onto the land. Known as saltwater intrusion, this occurs when storm surges or high tides over top areas low in elevation. It also occurs when saltwater infiltrates freshwater aquifers and raises the groundwater table below the soil surface.
|
|
What are isotopes?
|